전 세계적인 코로나19의 확산으로 일상화된 비대면 생활 속에서, 환자와의 직접적인 접촉이 불가피했던 병·의원의 각종 진료 방식에도 다각적 변화가 필요해졌다.
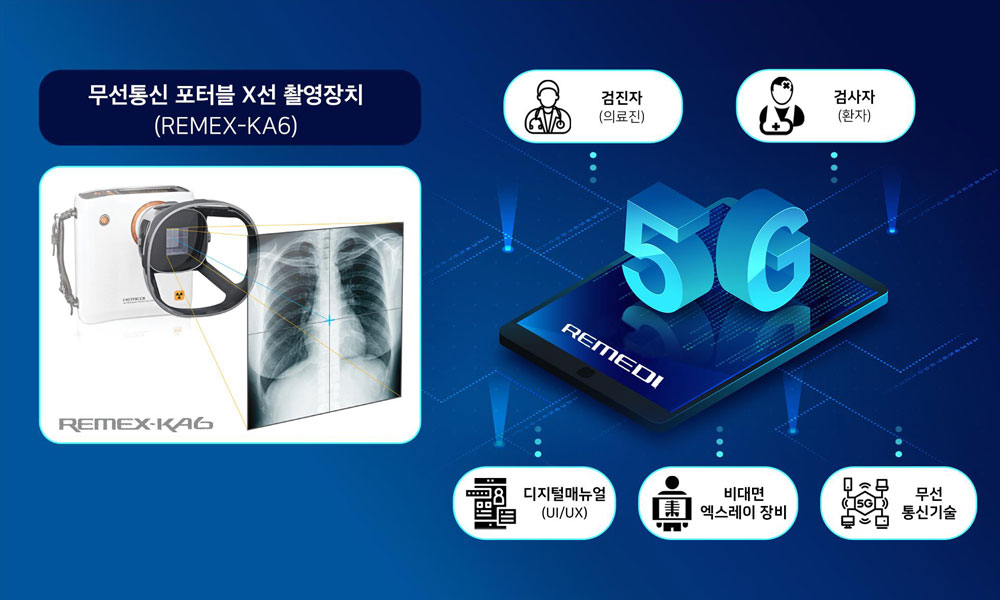
방대한 데이터를 처리하기 위해 설치형 영상진단장비에 의존할 수밖에 없었던 X-선 촬영에 주목한 ㈜레메디는 5G 환경을 기반으로 한 포터블 X-선 촬영장비와 X-선 촬영 데이터를 처리할 수 있는 애플리케이션을 개발, 공간적 제약을 가장 크게 받았던 X-선 촬영을 무선으로 진행할 수 있는 길을 열었다.
레메디는 의료진과 확진자의 접촉을 최소화 할 수 있는 X-선 검사기기 개발로 코로나 선별진료소와 생활치료소 뿐만 아니라, X-선 검사를 필요로 하는 모든 환자와 의료진에게 보다 편리하고 안전한 검사 환경을 제공하고 있다.
방사선 기술혁신을 통한
인류의 삶의 질 향상
방사선 의료기기 서비스의 접근성 향상 및 고객의 니즈에 부응하는 고부가가치 제품 개발의 필요성을 인지한 레메디는 ‘방사선 기술혁신을 통한 인류의 삶의 질 향상’이라는 비전과 함께 방사선 영상진단 의료기기의 대중화를 실현시키기 위해 설립되어, 방사선 진단검사에 관한 미래 수요에 대응할 수 있는 원천 기술 연구에 주목했다.
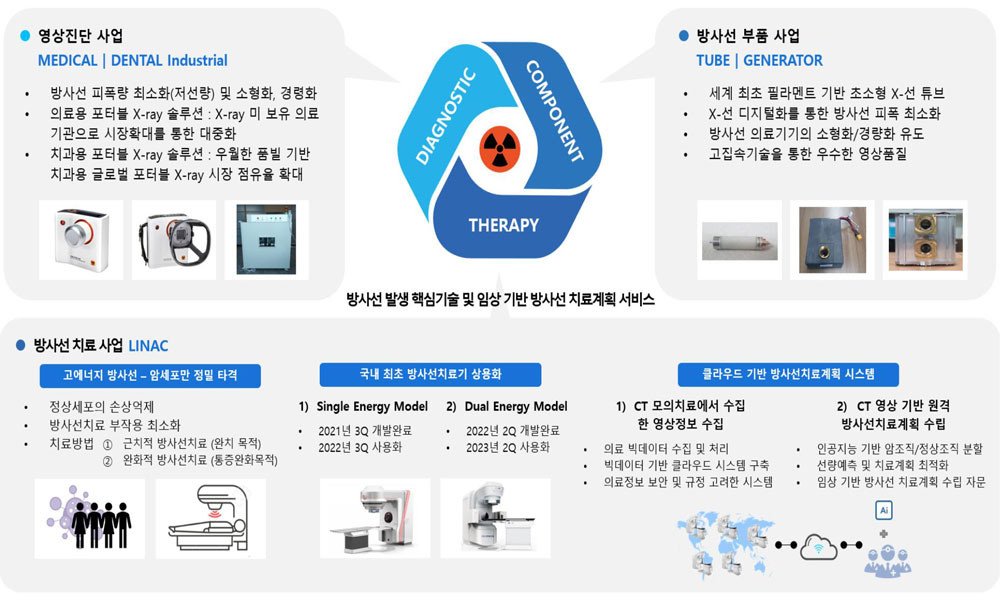
수준 높은 방사선 기술과 다양한 임상경험을 기반으로 디지털 헬스케어 토탈 솔루션 분야의 혁신을 주도하고 있는 레메디는 초소형 X-선 진단기기 부품과 소재, XIT 플랫폼의 핵심기술을 통해 글로벌 시장을 선도하고 있다.
XIT 플랫폼 기술력, 소형화 노하우, 신속개발 능력이라는 핵심 경쟁력과 가격 경쟁력을 바탕으로 X-선 영상촬영 및 치료산업 분야별 최적의 시스템을 제공하고 있으며, 비대면 시대에 꼭 필요한 포터블 X-선 촬영기기를 개발, 보급함으로써 방사선 진단기기 업계 안팎의 높은 관심을 받고 있다.
비대면 시대에 꼭 필요한
포터블 X-선 촬영기술의 개발
레메디에서 개발한 포터블 X-선 디지털 카메라와 Handheld X-선 카메라는 고정된 X-선 진단기기 위에서 피검자가 자세를 바꿔 가며 촬영해야 했던 종래의 인식을 깨트리고, 휴대용 카메라로 촬영하듯이 손쉽게 촬영할 수 있는 높은 자유도를 제공한다.
포터블 X-선 촬영기기를 활용하면 원하는 영상 각도를 찾기 위해 피검자의 자세를 힘겹게 바꿀 필요가 없으며, 코로나와 같이 검사자와 의료진 간 접촉을 최소화해야 하는 선별진료소, 생활치료소와 같은 공간에서도 손쉽게 검사할 수 있어 큰 주목을 받고 있다.
또한, 비대면 사용환경에 적합한 디지털 콘텐츠를 개발하여 코로나19 진단에 필수적인 흉부 X-선 촬영 및 비대면 검사 시스템을 구현하였으며, 사용자에게 디지털 매뉴얼 및 영상콘텐츠가 포함된 애플리케이션을 제공하여 편의성을 더해주고 있다.
미래 의료 서비스의 방향 제시로
디지털콘텐츠산업 활성화를 주도하다
앞으로 레메디는 사용자의 편의성에 중점을 둔 인터페이스를 개선해 나아가며, 피검자와 의료진 간에 코로나19를 비롯한 기타 전염병 역시 감염의 우려 없이 빠르게 진단할 수 있는 기술을 만들어 갈 계획이다.
2018년 강원도 유망중소기업 선정, 2019년 강원수출대상 은상 수상, 2020년 기술혁신형 중소기업(Inno-Biz) 인증 및 2021년 중소벤처기업부 수출유망 중소기업 선정 등 해마다 괄목할만한 족적을 남기고 있는 레메디.
비대면 시대에 최적화된 사용자 편의성을 우선으로 미래 의료 서비스의 방향을 선도해가며, 정확하고 편리한 X-선 촬영장치 개발 분야에 거듭 새로운 역사를 기록해 나아갈 것으로 기대된다.
미니 인터뷰
Q1. 이번 사업 참여로 얻은 성과는 무엇인가요?
“무선통신 기술을 활용하여 X-선 촬영장치와 연동 가능한 애플리케이션 및 프로그램을 개발하였습니다. 키오스크(선별진료소 같은 개념)와 연동할 수 있는 애플리케이션으로 비대면 사용환경에 적합한 X-선 검사 시스템을 구현하였으며, 관련 병원, 대학과의 독점판매 계약을 체결하였습니다.”
Q2. 성과를 낼 수 있었던 성공 비결은 무엇인가요?
“코로나로 인한 시장환경의 변화를 예측하여, 높은 품질 경쟁력을 바탕으로 미래 수요에 발 빠르게 대비 했기 때문이라고 생각합니다. 아울러 지속적인 연구개발을 통해 피폭량을 대폭 감소시키고, 이동 편의성까지 갖춰 실제 상용화 단계까지 이룰 수 있었습니다.”